
Medicine Cabinet
By Teodoro B. Padilla
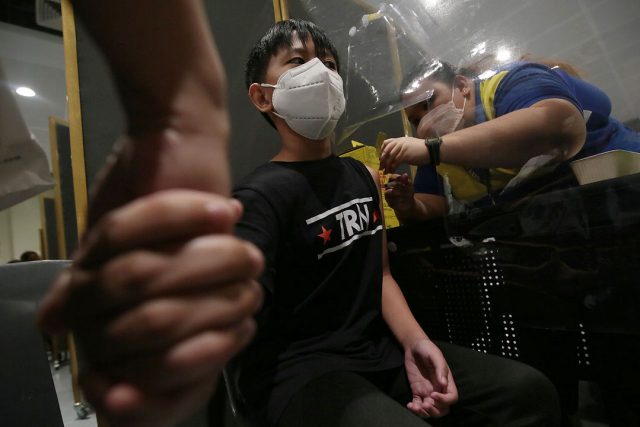
The Department of Health (DoH) and the National Task Force Against COVID-19 (coronavirus disease 2019) said that the policy to vaccinate children in the 5 to 11 age group is the result of careful study by health experts and has been approved in many countries.
In a joint statement, they said that more than 8.1 million children have been vaccinated worldwide, with no reports of deaths and serious adverse events among those vaccinated.
Vaccinating children aged 5 to 11 years old prevents severe COVID-19, hospitalization, and serious and long-term complications.
Citing modeling data from the European Center for Disease Prevention and Control, the Philippine Pediatric Society (PPS) and the Pediatric Infectious Disease Society of the Philippines (PIDSP) stated in their joint statement that vaccinating children aged 5 to 11 years old could also reduce COVID-19 transmission in the whole population, lower the likelihood of school absences and closures, lessen interference with social activities, and ease stress on families affected by COVID-19 and its associated disruptions.
PIDSP president Dr. Mary Ann Bunyi, also a member of the National Immunization Technical Advisory Group (NITAG), urged parents to have their children in this age group vaccinated when the government’s pediatric vaccination program, which started on Feb. 7. With parental consent, the DoH has received at least 100,000 pre-registrations for the vaccination rollout for the said age group.
“The vaccine is safe and effective. This is an opportunity for your family and children to be protected against COVID-19. Getting fully vaccinated will enable your children to go outside the house, attend face-to-face classes, and play with their friends,” she said.
In December 2021, the Philippine Food and Drug Administration issued an emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine for children aged 5 to 11 years. The vaccine for this age group has a lower dosage than the vaccine for the 12 to 17 age category and adults. The government aims to vaccinate at least 13.5 million children aged 5 to 11 against COVID-19.
“Published data from randomized clinical trials for the Pfizer-BioNTech vaccine showed that a vaccination regimen consisting of two 10-μg doses of the vaccine administered 21 days apart among 5–11-year-olds had a favorable safety profile and antibody levels comparable to those in 16-to-25-year-olds. A vaccine efficacy of 90.7% has formed the basis of approval for use of the vaccine in the Philippines and in other countries,” according to the PIDSP and PPS joint statement.
It added that safety data from United States surveillance showed that serious adverse events following Pfizer-BioNTech vaccination in children ages 5 to 11 years occurred in only 2% of recipients, with fever and vomiting as the most frequently reported. In the latest Philippine safety data for the 12-17 age group, dizziness, injection site pain, fever, increased blood pressure, and headache were the most commonly reported adverse reactions.
Protecting children from COVID-19 is crucial. The PPS and PIDSP noted that in the Philippines, around 3% of COVID-19 cases and 0.5% of deaths are among children aged 5 to 11 years old, which is similar to the worldwide trend. “Although COVID-19 cases in children remain less severe compared to older adults, children can still be hospitalized and even require admission to the intensive care unit (ICU).”
Moreover, the two societies said that between 0.5% and 3.1% of all children diagnosed with COVID-19 and between 0.9% and 7.6% of hospitalized pediatric COVID-19 patients may be at risk of developing multisystem inflammatory syndrome in children (MISC). MISC is a condition where different body parts can become inflamed, including the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal organs.
“[Children] may also be at risk of developing ‘long COVID’,” the PPS and PIDSP stated. Also known as long-haul COVID or post-acute COVID-19, long COVID refers to a wide range of new, returning, or ongoing health problems people can experience four or more weeks after first being infected with the virus that causes COVID-19.
The US Centers for Disease Control and Prevention said that people with long COVID commonly report experiencing different combinations of the following symptoms: difficulty breathing or shortness of breath, fatigue, symptoms that get worse after physical or mental activities (post-exertional malaise), difficulty thinking or concentrating (sometimes referred to as “brain fog”), cough, chest or stomach pain, headache, fast-beating or pounding heart (heart palpitations), joint or muscle pain, pins-and-needles feeling, diarrhea, sleep problems, fever, dizziness on standing (lightheadedness), rash, mood changes, change in smell or taste, and changes in menstrual period cycles.
Teodoro B. Padilla is the executive director of the Pharmaceutical and Healthcare Association of the Philippines (PHAP), which represents the biopharmaceutical medicines and vaccines industry in the country. Its members are at the forefront of research and development efforts for COVID-19 and other diseases that affect Filipinos.